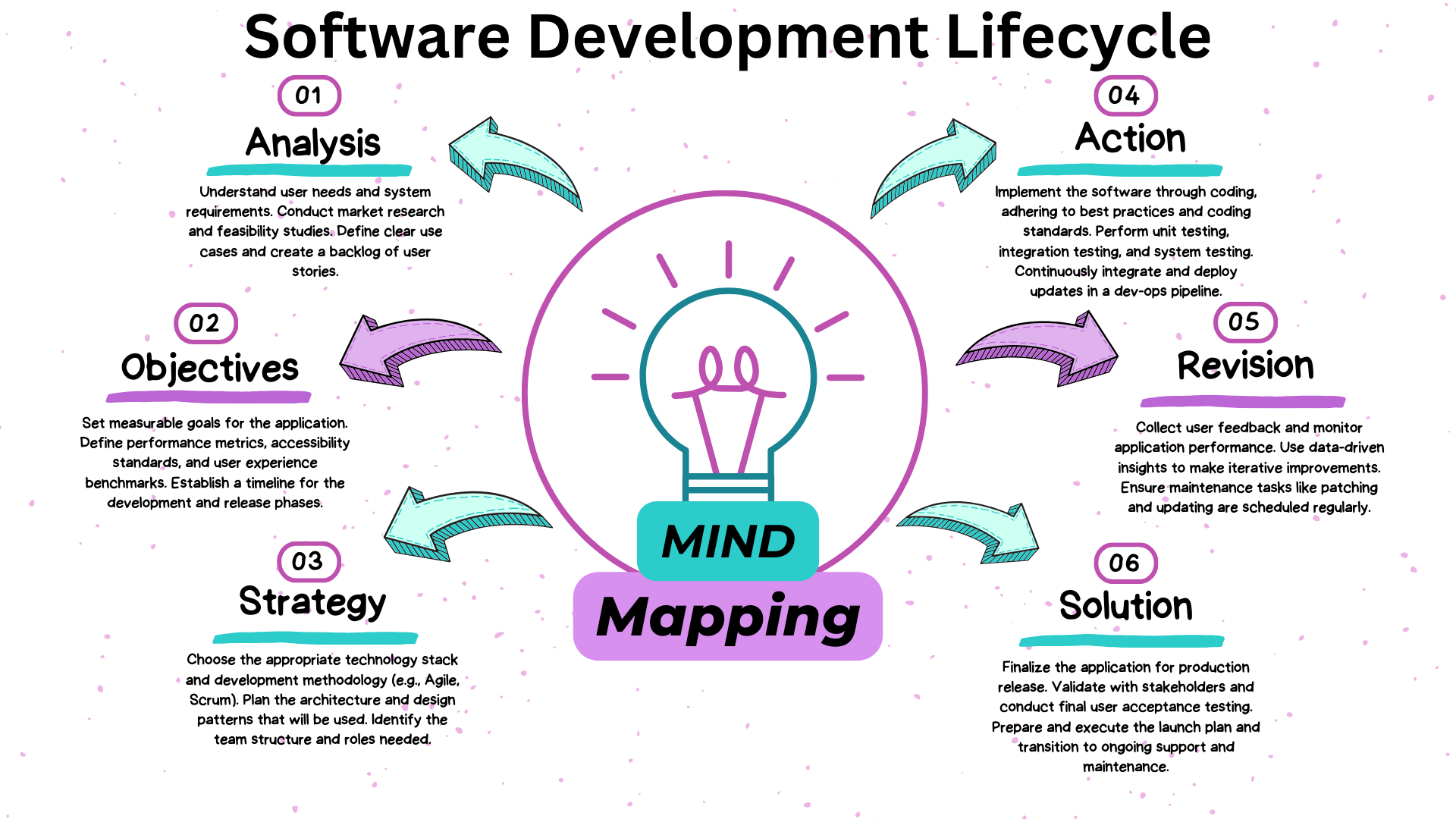
CRM Solutions for Pharmaceutical and Clinical Research
CRM Solutions for Pharmaceutical and Clinical Research
organizations (CRO's) can be used as a proven success model.
Salesboom on-demand Cloud CRM software solution, offers front
and back end integration for efficient end-to-end management
software for order and supply activities in addition to
facilitating investigator relationships, results from research
associates, and tracking of clinical trial status.
Contents:
Salesboom.com is a leading provider of cloud-based CRM solutions built specifically to the life sciences industry.
With Salesboom Cloud Based CRM Solutions, pharmaceutical enterprises are enabled by technology – not limited by it.
Since 2003 Salesboom.com enables pharmaceutical reps to use the CRM Solution anywhere through the Internet.
Also, managers can instantly monitor activities in the field with robust reporting.
Salesboom Cloud Based CRM software manages marketing pipeline, sales processes and monitor lead generation process, and all other automated marketing needs for pharmaceutical companies of any size. Our software solution is scalable, flexible and used by over 1,500 businesses worldwide.
Pharmaceutical corporations are in severe competition to get into the doors of doctor's offices and hospitals. Salesboom Cloud CRM Solutions such as lead management that helps reps build relationships and stay competitive.
The pharmaceutical industry is faced with more fluctuations and challenges than before, the patent cliff, increased regulations, and pricing pressures, to name a few. These unmatched changes are forcing corporations to do more with less and educate the client more effectively.
In a rapidly changing environment, it’s getting harder for pharmaceutical enterprises to rapidly adapt their sales and marketing models to achieve this. That is, unless they have the right tools and solutions such as: The CRM Software Solution.
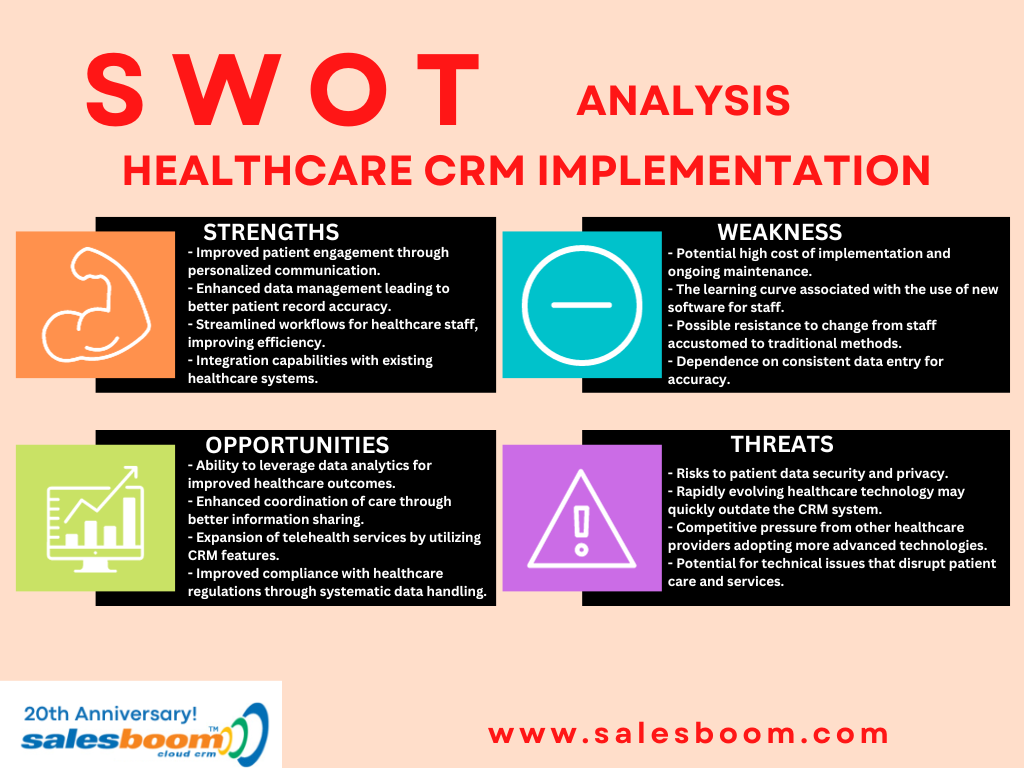
Salesboom can deliver Cloud CRM System Software solutions that enhances and tracks information sharing for team members in an on-demand environment. The result is improved communications, timely response to information, better efficiency without costly delays and/or errors in support of clinical research activities.
Stable Cloud CRM system solutions can integrate and streamline complex interaction between physician investigators, research teams, and administrative functions such as sales, marketing in the clinical research process and transitions from investigation to prescribing.
It is possible to realize a robust use of information and resources to enhance decision making and further efforts on time-to-market with the use of Salesboom Cloud CRM System software solution.
Secure Cloud CRM System Information Exchanges
Protecting information is priority in a clinical research environment is paramount. Salesboom Cloud CRM software solution assures tight access control to information and data stores through implementation of permission protocols. The protocols are based on set rules for access to information, and are implemented according to your organizations access rules. The software allows modification only at the highest level in your organization.
Access to information is based on a need to know. Salesboom Cloud CRM software system includes an audit option available in real time to ascertain access and/or changes to any information item in the system, any time, over time. With access control, you secure the information exchange and with audit functionality you can review the information item any time to determine changes, access patterns and demonstrate adherence to information and regulatory protocols for added protection.
Continuous Improvement
Salesboom Cloud CRM Software system solution is scalable, can harmonize system and business process activities in clinical research organizations as improvements are identified and changes implemented.
In acquiring flexible Cloud CRM software solutions, it is possible to pursue a policy of continuous improvement without an application constraint.
Salesboom Cloud CRM system software delivers rapid configuration, supporting existing, efficient sales processes and ability to meet the demands of a highly mobile clinical team.
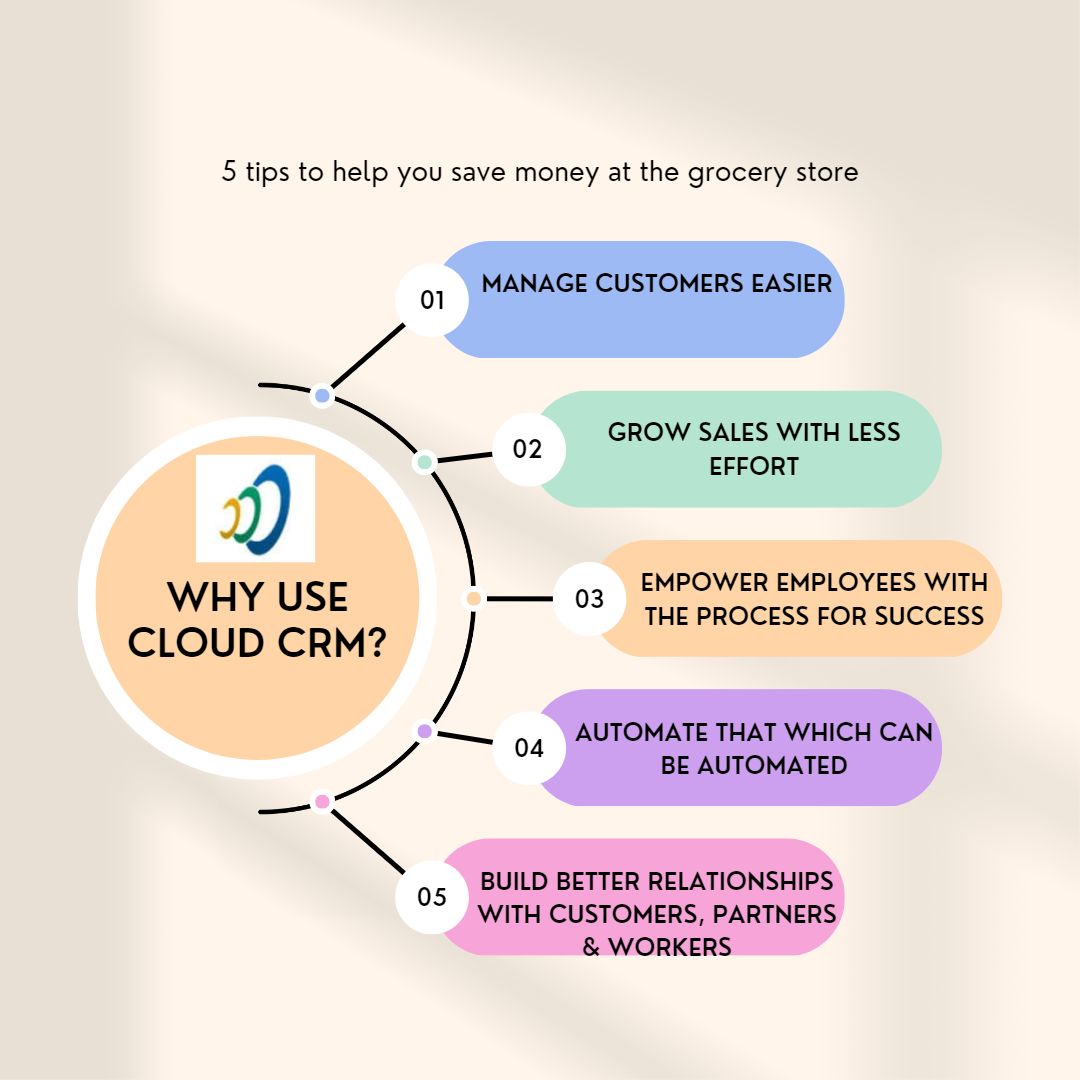
Because Salesboom offers user customization capacity, it is possible to enhance any tab to incorporate very specific business activities. You can develop templates to monitor subject schedule/visits, format reporting templates and enhance call-center activities as needed.
Regulatory Compliance
Salesboom Cloud CRM software system solution integrates rules based triggers that will flag any member(s) of the team to indicate a required action to meet and maintain compliance for regulations specific to clinical research activities. There are a number of ways to accomplish this depending on the business rules in place within the clinical setting.
With on-demand access to information and protocols across the organization, better decision making can be supported and significant errors can be avoided.
Professional Services - Cloud CRM Custom Solutions
Salesboom Cloud CRM system software and Professional Services address specific requirements for the clinical research organizations and delivers on demand solutions that automate processes on time and within budget.
Salesboom has evolved solid solutions based on dialog and responsiveness to customer needs.

Salesboom offers a stable, scalable software solution with a successful deployment based on support and flexibility of our stand-alone and custom integrated Cloud CRM Software Solutions for Enterprise Resource Planning (ERP) and support and training services offered as part of our implementation.
Salesboom Cloud CRM System Software will deploy a solution that enhances services and processes so you can achieve positive ROI in as short a time as possible:
- Software Solution – our team will help develop Cloud CRM System plan, strategic placement and requirements
- development
- Identify and automate business processes
- Software and/or process customization
- Integration of Cloud CRM Software (with existing software)
- Cleanse/Import Data
- Reporting on implementation/deployment
- Professional Training Services and Support Services
The Salesboom Platform:
- Web Services with Service Oriented Architecture (SOA)
- Optional Customization - Capacity for user or professional customization and is fully extensible
- Development and Integration - Web Services API and AJAX / COMET controls are system standards.
- Cost control - costs are contained because the platform is proven both flexible and stable
- Partner Networks - An extensive partner network of over 125 different solutions providers
- Legacy - Salesboom has an API that facilitates integration for web based Cloud CRM/ERP solutions with
- existing front and back office systems.
Professional Services
Salesboom offers professional services in support of the Cloud CRM software system implementation life-cycle and beyond. You might consider:
- Business Modeling Services
- Project Management (PM) Services
- Professional Data and System Analysis
- Custom Integration Services
- Support and Training Services
CRM systems empower pharmaceutical companies to manage relationships and automate sales processes, delivering excellent customer experiences with a higher degree of accuracy. By reducing data entry errors, leveraging workflow rules and custom fields, integrating with other applications, and fostering team collaboration, these CRM solutions optimize sales performance, enhance forecasting accuracy, and enable the delivery of personalized and timely interactions at each stage of the sales journey.
Check out our new CRM System For Pharmaceutical Companies ClinicalTrials.gov from here: https://salesboom.com/pharmaceutical-crm-contracts-projects-webforms-clinicaltrialsdotgov.html
